Stent Failure – Insights from OCT imaging.
Thank you again, the kind introduction. My talk today here is on stent failure. This is my disclosure. As you know, imaging is recommended to identify the stent-related mechanism of Class IIa based...
Thank you again, the kind introduction. My talk today here is on stent failure. This is my disclosure. As you know, imaging is recommended to identify the stent-related mechanism of Class IIa based on the ESC guideline, as you know very well.
So, let's start the case. This is a 63-year-old male, STEMI. We put the stent at the acute phase. So the result is relatively poor but acceptable, but the OCT imaging on the right demonstrates lots of thrombus protrusion as shown in this right-hand side movie. Right here... lots of protrusion. This is in still frame. Lots of red thrombus protrusion. At that time that you will use a IIb/IIIa inhibitor but in Japan we do not have a IIb/IIIa inhibitor. It is not available. Then we just follow it.
13 days after a primary PCI, the patient had a chest pain suddenly and then angiography demonstrate occlusion and then you can identify the organized thrombus in the distal portion and still lots of thrombus protrusion in the proximal side. Then we did a thrombus aspiration. It looks a fair result therefore, we decided to put the bare metal stent because the patient may have some allergy to the drug coated stent. Then we put and then acceptable result, but not enough.
This in the OCT imaging still, even after additional bare metal stent, there are lots of thrombus protrusion. Again, you would feel like a IIb/IIIa inhibitor but we thought at that time, they're a poor metabolizer of the Clopidogrel. Then we also checked the condition. This is the result, genotype 2/2, it's a poor metabolizer. So at the same time, we changed Clopidogrel to the Prasugrel to maintain the patency of the stent. This is six months after stenting, after sub-acute stent thrombosis.
Heterogeneous tissue growth can be identified and some peri-strut hollow but the is result is acceptable. Some stent protrusion to the bifurcation lesion. It should be acceptable. There are lots of mechanism of acute or sub-acute stent thrombosis. This is already proposed at the time of bare metal stent but also we have to think about anti-thrombotic therapy. It might be one of the causes of acute or sub-acute stent thrombosis.
So let's start another example of the very late stent thrombosis. Patient had the SES in the proximal LAD. Seven years after the initial ECI, he suffered with chest pain and with very late stent thrombosis. So, an EES was implanted into it. We tried to show you the six month follow up and the OCT imaging and 19 months follow up. Here is at the time of stent thrombosis, very late stent thrombosis.
We did an... Aspiration and then the result is acceptable. This is an OCT imaging at the time of very late stent thrombosis. There are lots of acquired incomplete apposition, and also the peri-strut hollow. I'm not sure the mechanism, we luckily have an OCT imaging six month followup at the very late stent thrombosis. At that time, the result is yes, acceptable. There are still some peri-strut hollow and also the heterogeneous tissue growth and also some incomplete apposition, but clinically it might be okay.
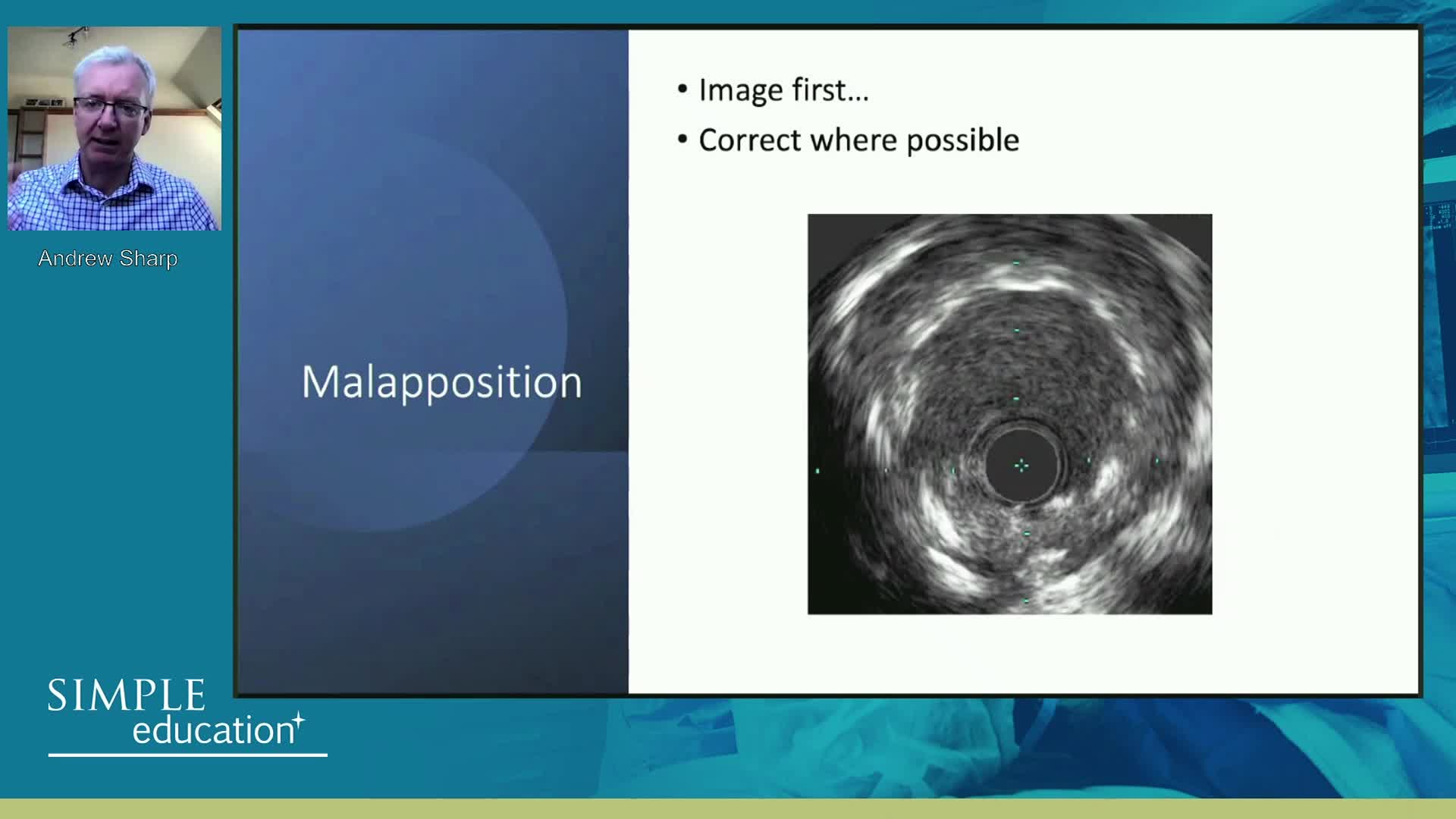
This time, 19 months after EES at the time of a very late stent thrombosis, later acquired mal-apposition increased much more and you can identify the rods. So incomplete apposition and peri-strut hollows. This may be the cause of very late stent thrombosis. We cannot identify any stenosis at the site of the stent at that time.
So the later acquired mal-apposition might be one of the cause of very late stent thrombosis. This is an IVUS data, I'll show you the recent OCT imaging data, so in case with stent mal-apposition... in case with late stent thrombosis, stent mal-apposition is significantly higher as shown in the right-hand side. Even in the mal-apposition, maximum lumen size and maximum length and mal-apposition depth is significantly greater in the late stent thrombosis with late stent mal-apposition. The mechanism is not clear, some allergic response to the drug coated balloon might be one of the mechanisms.
So before I show the late stenosis, I would like to show you another example of the stent problem. This is an acute coronary syndrome and then we put the stent. Then this is the result, but at that time you have to pay attention to the position of the guiding catheter. It's very close to the proximal side of the stent. IVUS demonstrate no stent in the proximal side and some stent in the other side. So the guide catheter makes some deformity and this is an OCT imaging finding the same case, some deformity. So you can identify the high density of blood in the second image and no fluid from three to seven in the right second. Some incomplete apposition. Therefore, the position of the guide wire is very important. You have to pay attention to the position of the guiding catheter when you treat the proximal region.
This is another case, we have to think about the shortening of the stent. This is a bifurcation lesion. So we did put the side branch guide wire to protect the site of arterial occlusion, we put the stent and after stenting the result is good, acceptable. Then when we take out the side branch guide wire, some resistance can be felt. Then this is the IVUS result. I will show you the OCT imaging result. Distal portion, we missed the stent strut, so the shortening of the stent was happen, we speculate. This is an OCT image, the same position. You can not identify any strut in the distal site. When we take out the wire, the shortening of the strut is what happened in this case. And then we put this stent in the distal portion to cover the region. This is the final result.
So there are lots of data about the... Longitudinal shortening or expansion. So let's move on to in-stent restensosis. This is a 74 years female. When she comes with unstable angina, we can identify instantly in-stent stenosis by angio. And then IVUS, this is already showed yesterday. IVUS can demonstrate the huge tissue growth, but we cannot identify what kind of tissue. This is an OCT image. It can identify very fibrous and some portion is in peri-strut hollows. This is a still frame, you can identify the heterogenous tissue growth. The distal side is very fibrous and the proximal side, the lower side is very good heterogeneous tissue proliferation. And then we did the ballooning. Then after the drug coated balloon, following the coated balloon, this is the result. We can identify the drug staining by OCT. You can identify clearly, so these are acceptable finally.
So there are some data about the in-stent restenosis. The left hand side, it's an ideal follow-up OCT findings, only a small amount of fibrous tissue covered the stent strut. This is a stable condition, however, sometimes tissue growth can be identified. This is a homogenous pattern, but that might be fibrous. Here, you can identify the significant poor stenosis. This is a heterogeneous, its an organization of the thrombus and this is the layer of the pattern.
There are no data showing one to one comparison between the OCT findings and the histology, but we can choose the treatment. This is the data from our colleague in Japan. If you identified homogenous pattern, of the tissue proliferation. There are coated balloon, Paclitaxel coated ballon is very effective compared with no drug coated balloon. If the backscatter is high, and the heterogeneous pattern there are no significant difference within the Paclitaxel... No, with the high backscatter, you can identify the better result of the Paclitaxel coated balloon. Heterogeneous pattern, there are no significant difference so you can save the drug coated balloon if you identify the tissue characteristics by OCT.
This is another case of in-stent restenosis. By follow-up you can identify the motion, hinge motion here. This is a kind of fracture. Then by IVUS, we can not identify any still at the mid portion right here, the B. OCT gives the same information. We can identify the strut in the distal and proximal and proliferation is maximum at the fracture site. So the loss of stent is sawed by OCT imaging, is demonstrating the stent fracture, but sometimes is enough to identify it as a fracture.
This is another case. The patient was bypassed previously, but the patient had an angina. Then we put a stent to the LCX, but again the patient had an angina because you can identify tissue proliferation by IVUS. OCT also gives you a very heterogeneous instant tissue proliferation in the proximal LCX. And then as I told you, a drug coated balloon is not so effective in this case, therefore, we use a scoring balloon and then the result is acceptable. This is the steel frame. You can identify the lots of cut portion in OCT. IVUS might be very difficult to identify such result.
This is another case, tissue proliferation within a stent and then the scoring balloon is very effective to obtain the good result as shown in this. If drug coated bottle is acceptable, we try to do the either drug coated ballooning at the site of in-stent restenosis, but it's dependent on the case.
This is in the very late restenosis, 11 years after bare metal stent. This is difficult to see because you can identify the tissue growth within the stent. However, there are lots of calcium, even in the proximal to distal. In the distal portion, you can identify very eccentric calcium within a stent. So simple ballooning is not enough to obtain the enough result in these cases. Tissue modification is recommended. In cases with very late stent thrombosis in bare metal stent or drug-eluting stent, you can sometimes identify a very thin fibrous cap as shown in this slide. It might be a neo-atherosclerosis
This is another case of a bare metal stent, a very late stent thrombosis. You can identify the thin cap fibro-atheroma and rupture of the thin cap fibro-atheroma where thrombus can be identified. This is a comparison of the late in-stent restenosis and the early in-stent restenosis thrombosis by OCT tissue characterization. Lipid-laden intima is very common in late stage and TCFA-like is very common and neo-neovascularization is very common in late in-stent restenosis. This is based on the neo-atherosclerosis accelerated neo-atherosclerosis in early in-stent restenosis. Such the findings is less neovascularization is relatively high.
As you know, this is a very famous, bare metal stent, neo-atherosclerosis starts nearly two years after stent implantation, but in the years you will need the early restenosis because of the accelerated atherosclerosis, as you know very well. So instantly neoatherosclerosis may be an important mechanism is DES failure. This is the recent data showing a mechanism on stent thrombosis in each period from acute to very late. This is an a prestigious organization in Europe. Based on this data, you can identify on the expansion in each period. This might be the course because this study is just used all city. At the time of event, they did not use OCT imaging at the time of in-stent implantation.
Therefore they did an angio guided PCI, but there are lots of even... And then at that time they tried to check on the OCT, what is the mechanism? So we cannot identify true mechanism, but we can identify what's really happened within a stent. So uncover the strut is quite often in acute and subacute stent thrombosis. Also mal-apposed strut is very common in acute and subacute. Yes, we can identify even in late and very late stent thrombosis. This might be the data acquired incomplete apposition, I speculate.
This is a very busy slide. You can focus on the left-hand side fast. In case with acute thrombosis, uncover the strut is the most frequent findings by OCT and also the mal-apposed strut, therefore incomplete apposition may be the main cause of acute thrombosis. Also, subacute similar findings can be identified, but you can also focus on under-expansion. So under-expansion also the mechanism of stent thrombosis, but the late and very late mechanisms should be completely different. There are lots of restenosis under-expansion, but still uncover the strut. This might be the acquired mal-apposition or something.
And very late, you can identify the lots of neoartherosclerosis road. This is a main cause, rupture of the neo-atherosclerosis might be the cause of very late stent thrombosis. They also compared each generations bare metal stent, generation one and generation two. In cases with bare metal stent, neo-atherosclerosis a main cause because we already have in the long time follow-up neo-atherosclerosis. Think of fibro-atheroma, makes a rupture and makes a stent thrombosis. This is a main mechanism, you can speculate without OCT imaging. But generation two, you can identify the lots of uncovered struts and also the mal-apposed strut that might be the cause of the stent thrombosis.
So this is my summary in stent failure. Although poor metabolizer of P2Y12 inhibitor might be one of the mechanism of acute or sub-acute DES, type of stent or polymer might relate to the stent thrombosis. Under-expansion, edge dissection, huge mal-apposition may lead acute or sub-acute stent thrombosis, and late acquired incomplete stent apposition, neo-atherosclerosis, uncovered strut with peri-strut hollow could be the mechanism of late and very late stent thrombosis.
Accelerated neo-atherosclerosis is thought to be a cause of early in-stent restenosis in DES, disruption of TCFA following slowly progressed neo-atherosclerosis might be the mechanism of very late stent thrombosis after bare metal stent implantation. OCT assessment of neo-intima tissue characterization might be useful to decide the treatment strategy and to predict the recurrence of in-stent restensosis and target lesion revascularization. Thank you very much for your time.
Speakers
A medical doctor at Wakayama Medical University, Wakayama, Japan. Features in 16 videos on... A medical doctor at Wakayama Medical University, Wakayama, Japan. Features in 16 videos on Cerebria. Takashi Akasaka generally speaks on Instantaneous wave-Free Ratio (iFR), Fractional Flow Reserve (FFR), Coronary Flow Reserve (CFR), Coronary Physiology, and Coronary Optical Coherence Tomography (OCT).